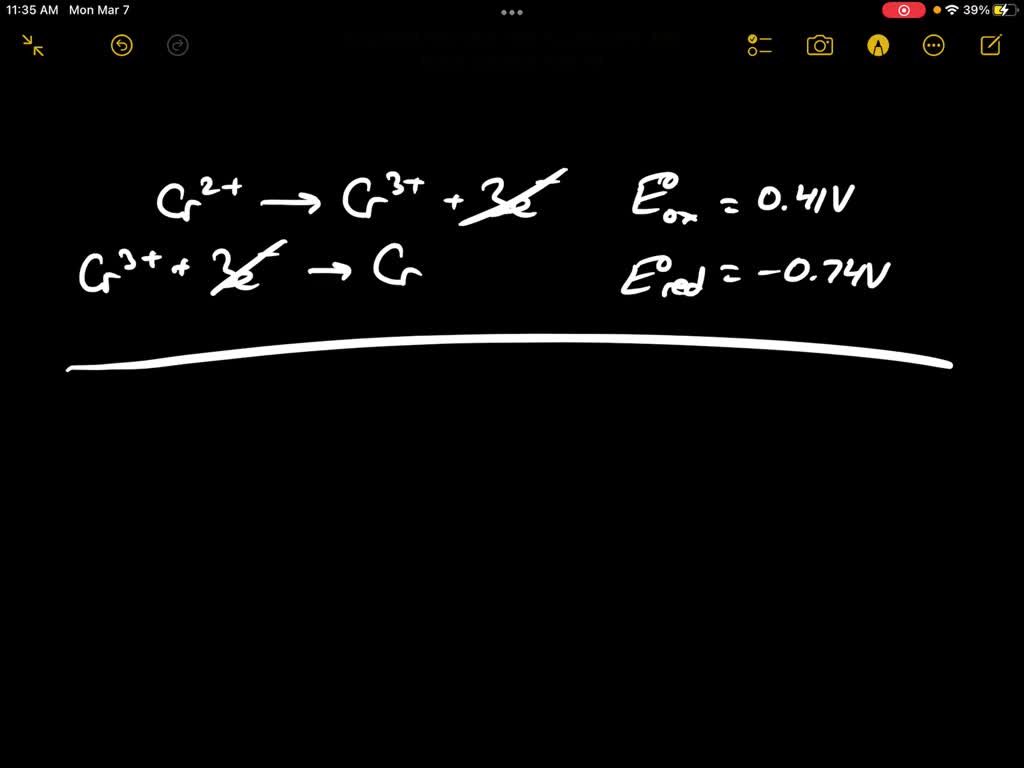
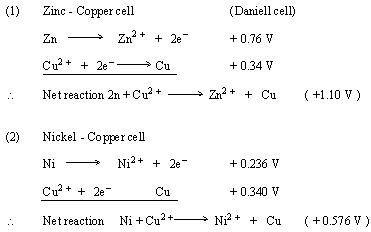
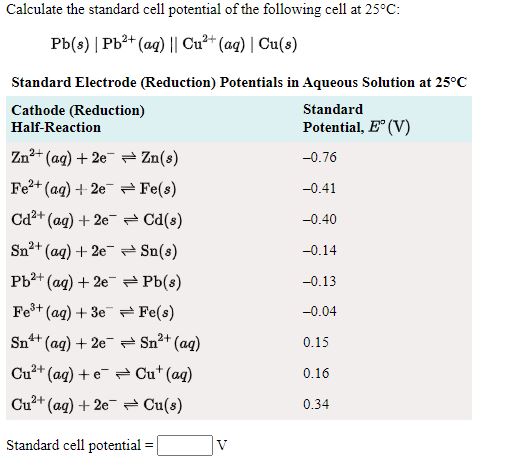
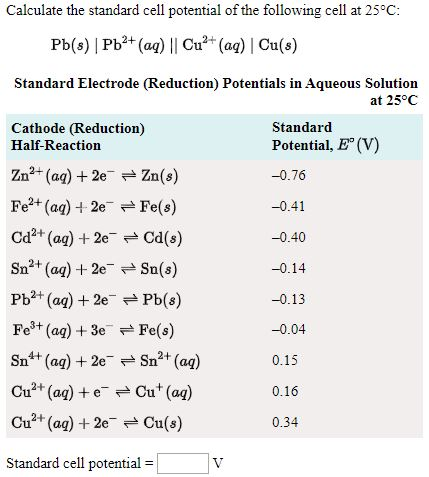
Calculate the standard cell potentials of galvanic cell in which the following reactions take place:(i) 2Cr(s) + 3Cd^2 + (aq) → 2Cr^3 + (aq) + 3Cd (ii) Fe^2 + (aq) + Ag^+ (

Standard Cell Potential: Calculations, Electron Flow & Feasibility (5.4.2) | CIE A Level Chemistry Revision Notes 2022 | Save My Exams

Calculate the standard electrode potential of the Ni^(2+)//Ni electrode , if the cell potential potential of the cell, Ni//N^(2+)(0.01 M)//Cu is 0.59" V ". "Given" E(Cu^(2+)//Cu)^(@)=+0.34 " V "

Calculate the standard electrode potential of the Ni^(2+)//Ni electrode , if the cell potential ... - YouTube

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa