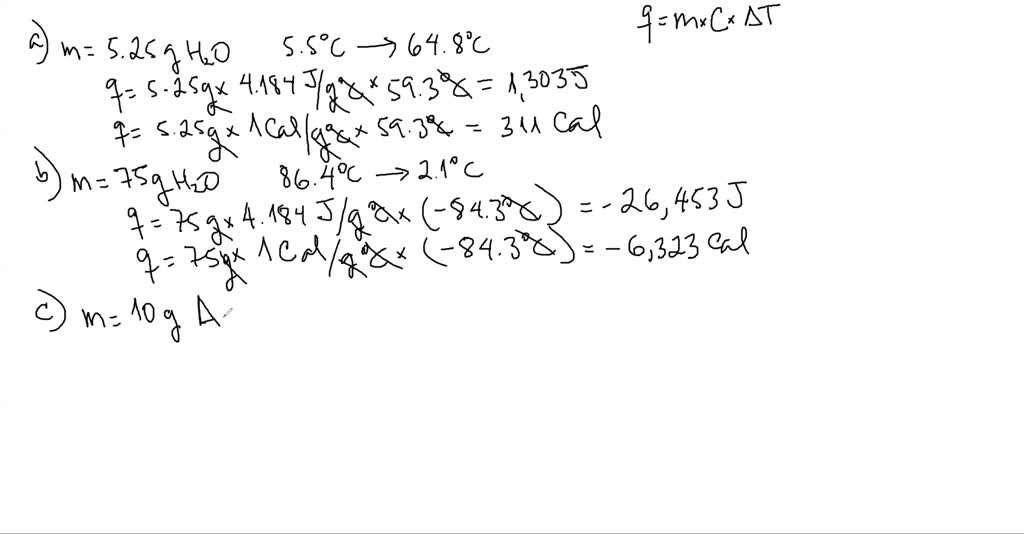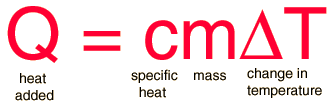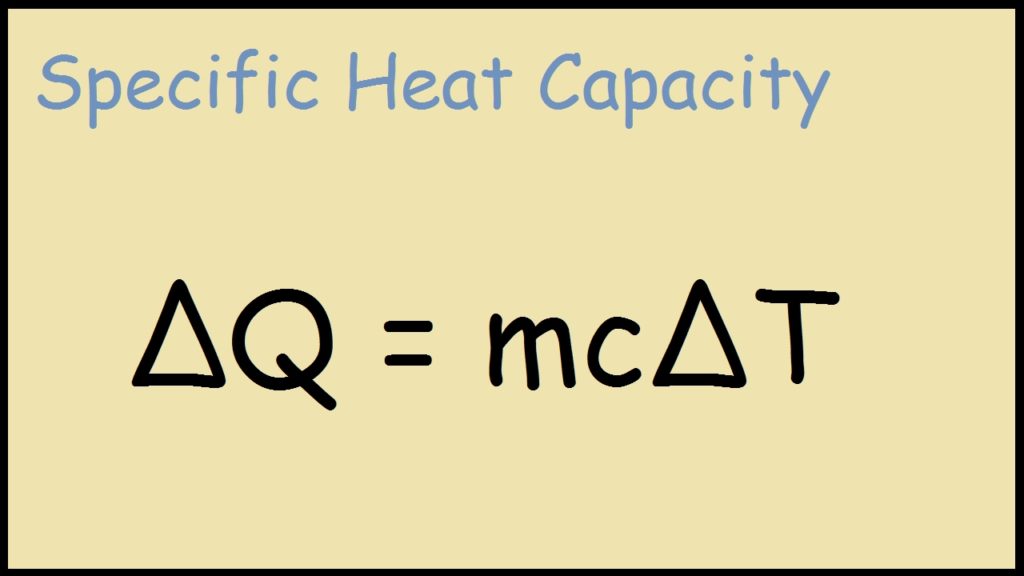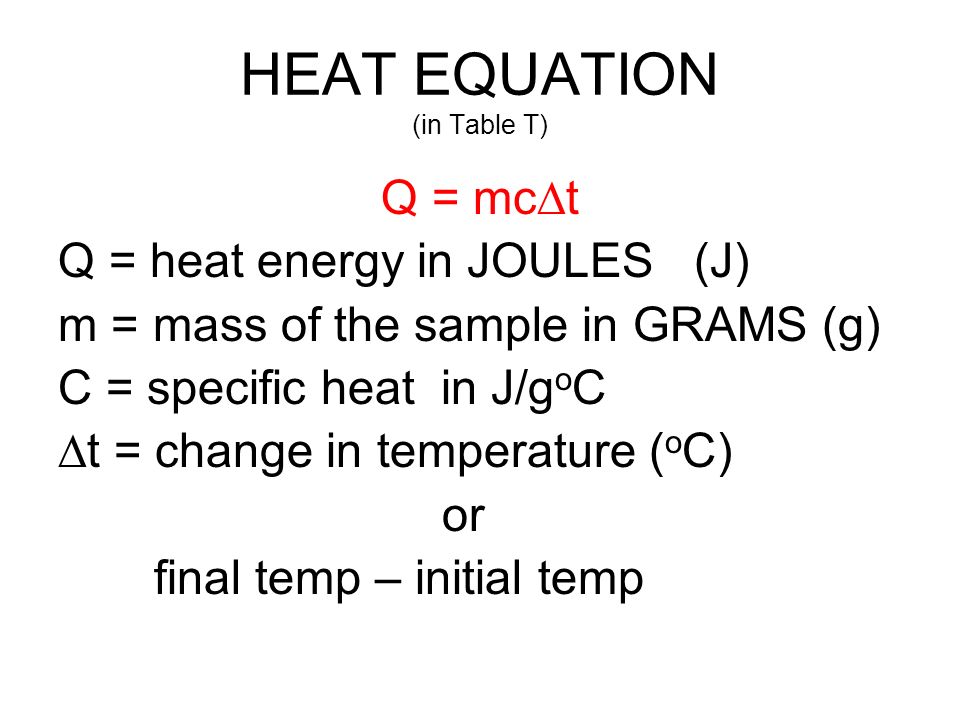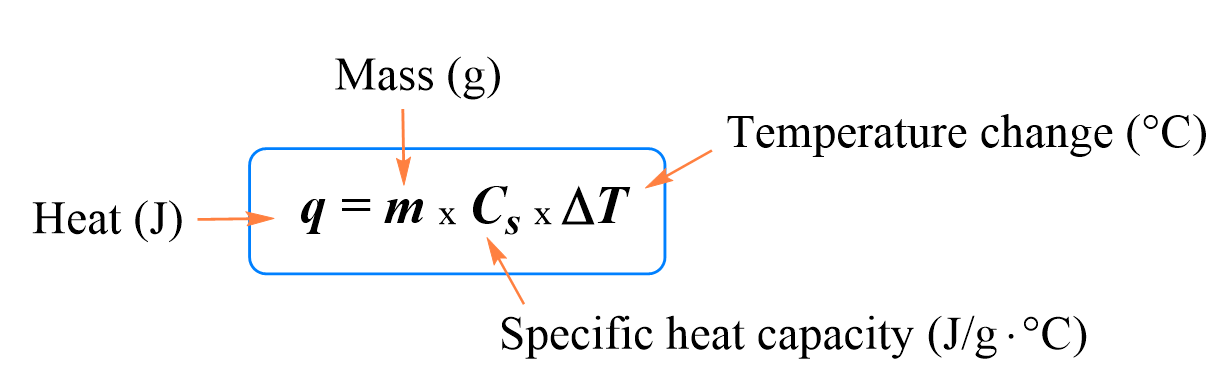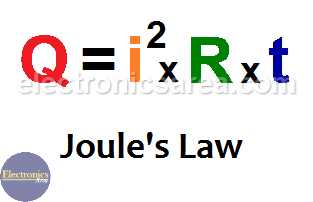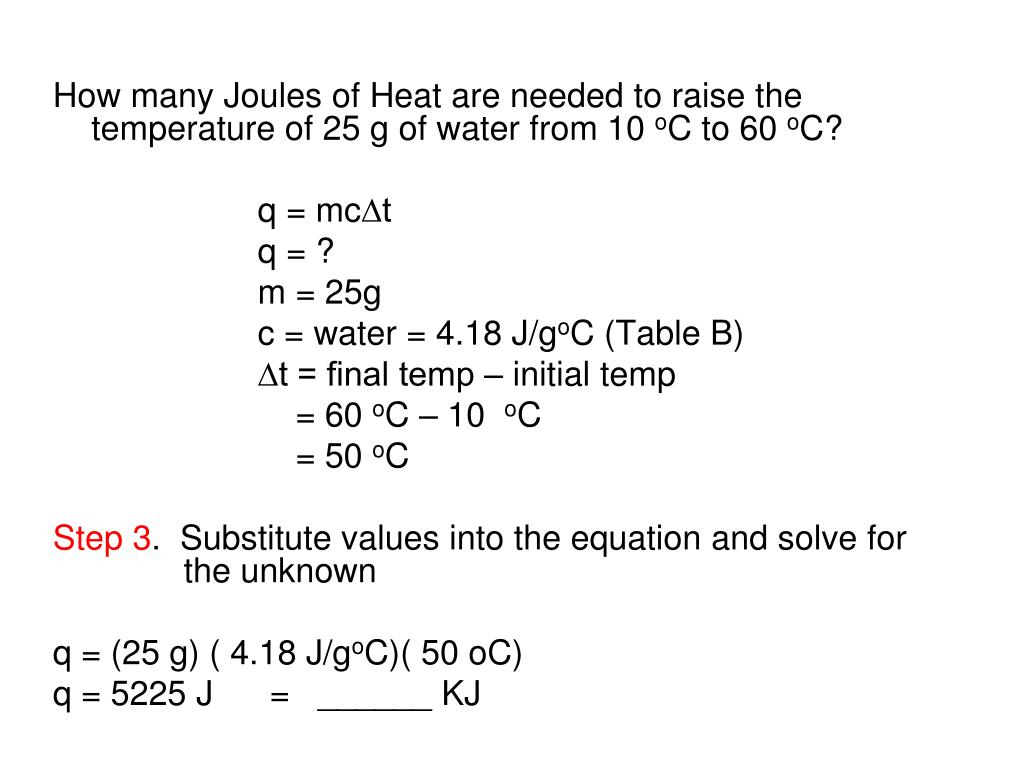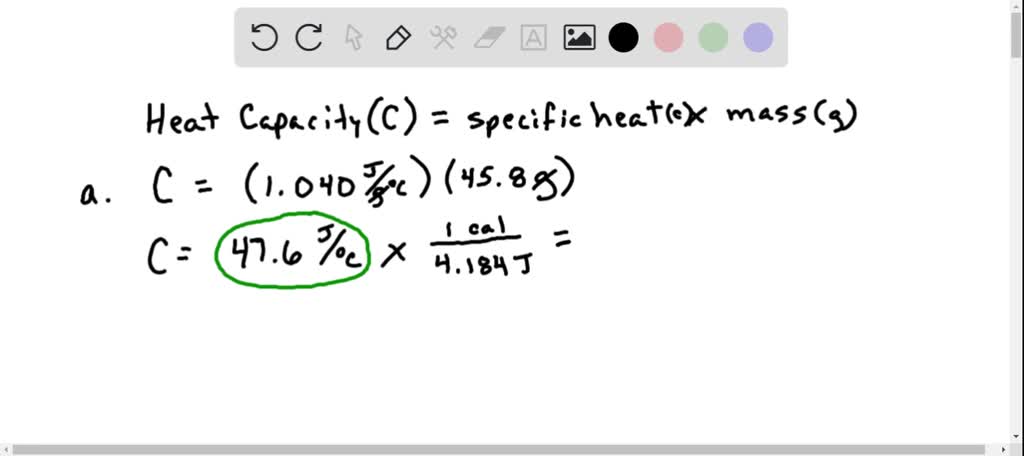
SOLVED:Calculate the heat capacity, in joules and in calories per degree, of the following: (a) 45.8 g of nitrogen gas (b) 1.00 pound of aluminum metal

Thermochemistry. Energy and Heat Energy = the ability to do work, measured in Joules (J) 1 joule = 1 Newton of force applied to a 1 kg object over the. - ppt download

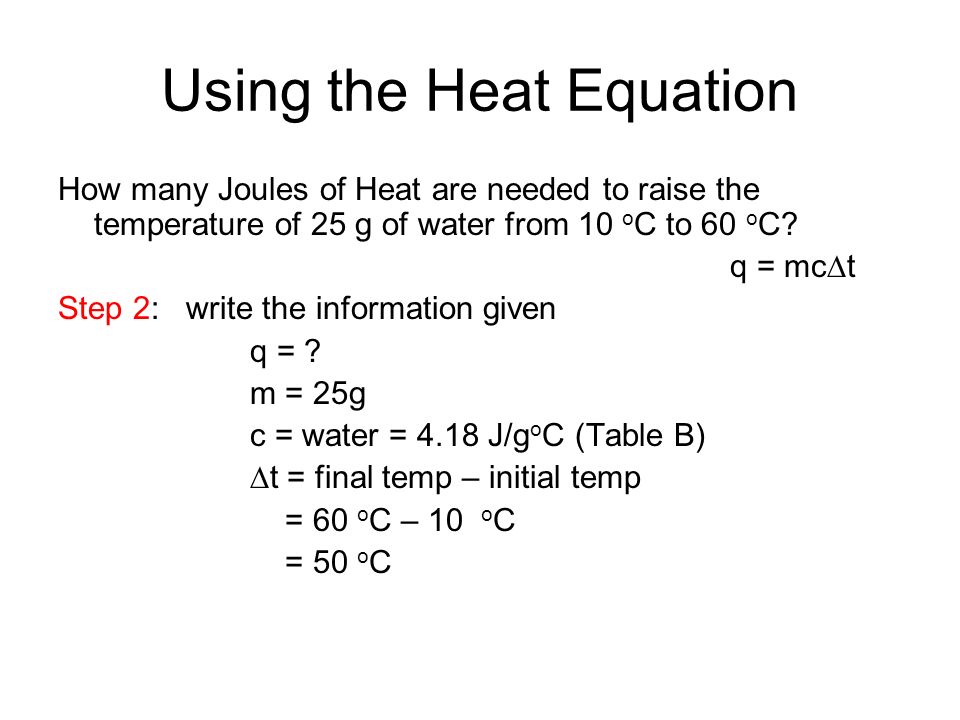
Calculate the amount of heat (in Joules) required to convert 25 grams of water at 100 ^∘ C into steam?(Given : heat of vaporization of water = 2257 J/g)