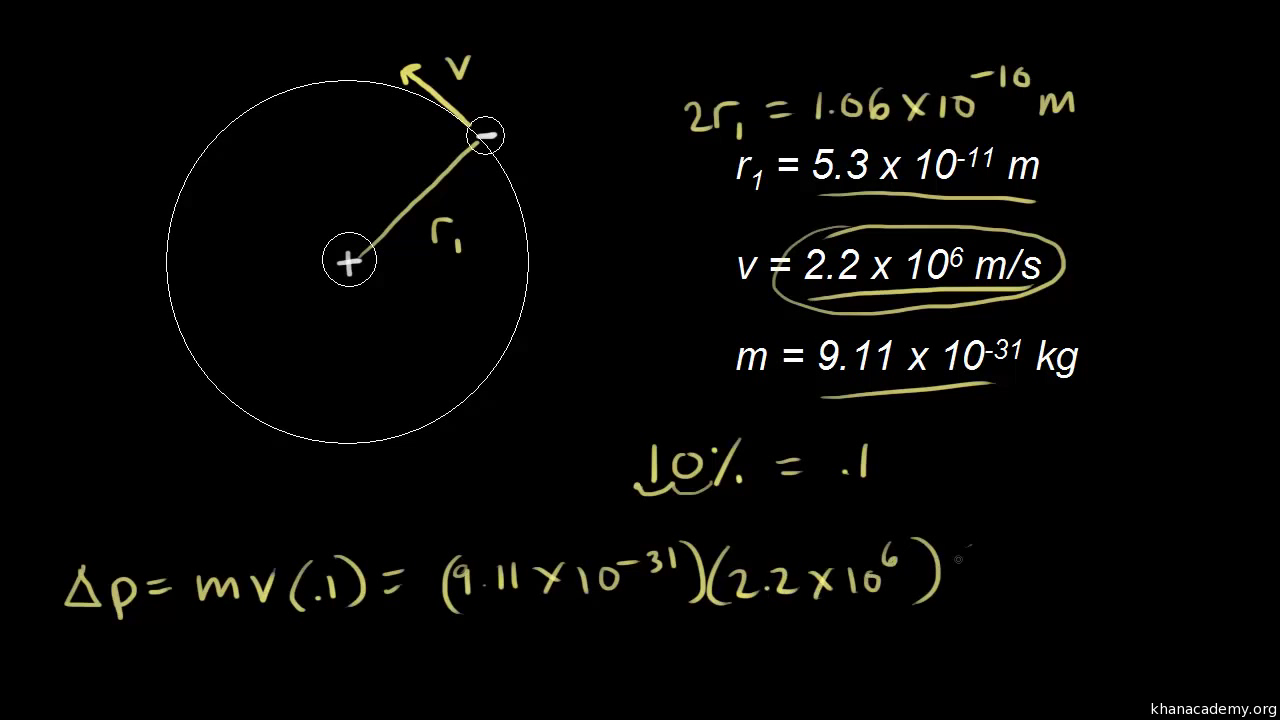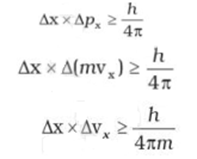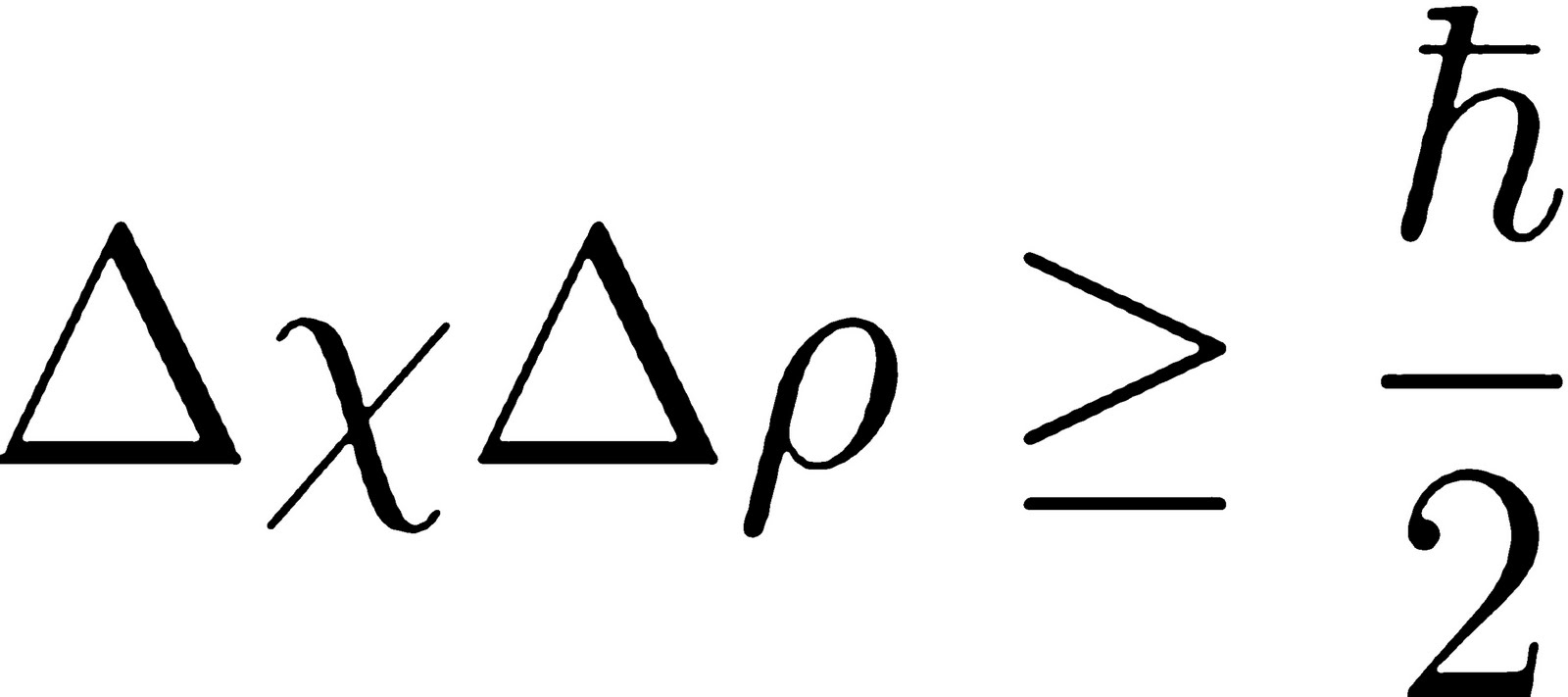![SOLVED: Heisenberg Uncertainty The Planck constant is 6.626*x104-34 ] The position of an object is measured t0 an accuracy of ( or with an uncertainty of) Ar = 0.0130 nm: (a) What SOLVED: Heisenberg Uncertainty The Planck constant is 6.626*x104-34 ] The position of an object is measured t0 an accuracy of ( or with an uncertainty of) Ar = 0.0130 nm: (a) What](https://cdn.numerade.com/ask_images/d8e1b903cb2045659164f4a50f8a5da7.jpg)
SOLVED: Heisenberg Uncertainty The Planck constant is 6.626*x104-34 ] The position of an object is measured t0 an accuracy of ( or with an uncertainty of) Ar = 0.0130 nm: (a) What

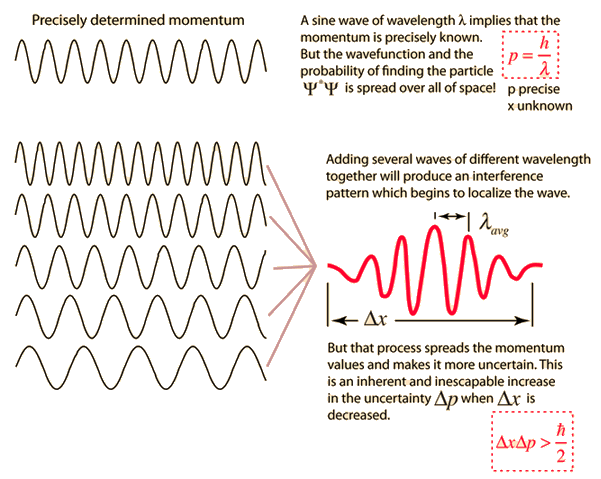
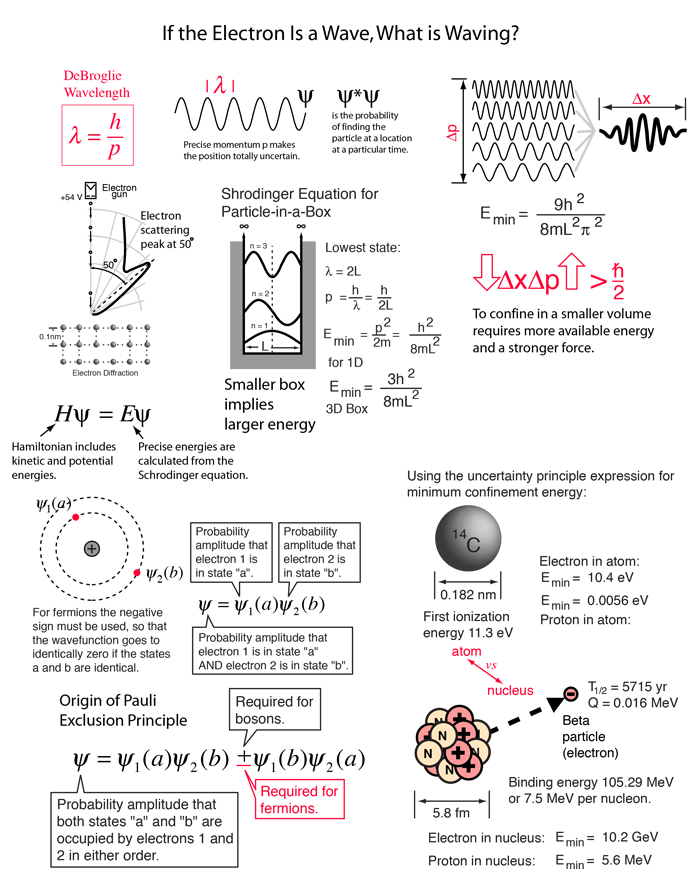
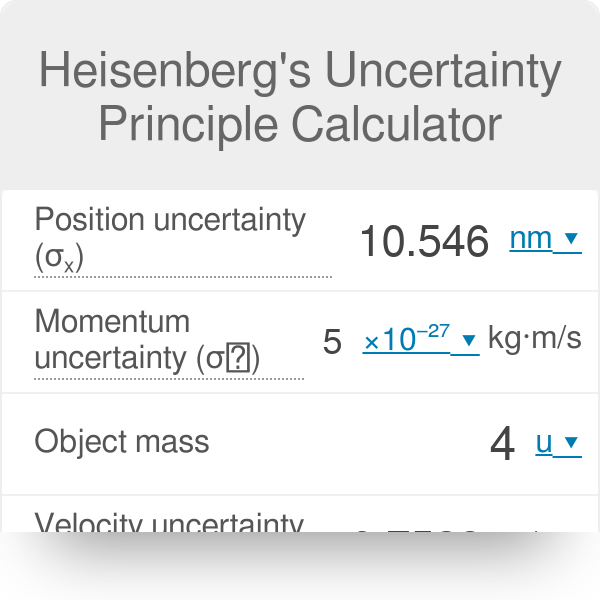
According to Heisenberg's uncertainty principle, the product of uncertainties in position and velocities for an electron of mass 9.1 × 10^-31 is
![SOLVED: Heisenberg Uncertainty The Planck constant is 6.626x10^-34 ] s. The position of an object is measured to an accuracy of ( or with an uncertainty of) Ax 0.0200 nm: (a) What SOLVED: Heisenberg Uncertainty The Planck constant is 6.626x10^-34 ] s. The position of an object is measured to an accuracy of ( or with an uncertainty of) Ax 0.0200 nm: (a) What](https://cdn.numerade.com/ask_images/99637fa1fa7946e7ad502688f413c006.jpg)
SOLVED: Heisenberg Uncertainty The Planck constant is 6.626x10^-34 ] s. The position of an object is measured to an accuracy of ( or with an uncertainty of) Ax 0.0200 nm: (a) What

Heisenberg's Uncertainty Principle Explained & Simplified - Position & Momentum - Chemistry Problems - YouTube